American Diabetes Association; 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2021. Diabetes Care 1 January 2021; 44 (Supplement_1): S125–S150. https://doi.org/10.2337/dc21-S010
Download citation file:
toolbar searchThe American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” includes the ADA's current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee (https://doi.org/10.2337/dc21-SPPC), are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA's clinical practice recommendations, please refer to the Standards of Care Introduction (https://doi.org/10.2337/dc21-SINT). Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
For prevention and management of diabetes complications in children and adolescents, please refer to Section 13 “Children and Adolescents” (https://doi.org/10.2337/dc21-S013).
Atherosclerotic cardiovascular disease (ASCVD)—defined as coronary heart disease (CHD), cerebrovascular disease, or peripheral arterial disease presumed to be of atherosclerotic origin—is the leading cause of morbidity and mortality for individuals with diabetes and results in an estimated $37.3 billion in cardiovascular-related spending per year associated with diabetes (1). Common conditions coexisting with type 2 diabetes (e.g., hypertension and dyslipidemia) are clear risk factors for ASCVD, and diabetes itself confers independent risk. Numerous studies have shown the efficacy of controlling individual cardiovascular risk factors in preventing or slowing ASCVD in people with diabetes. Furthermore, large benefits are seen when multiple cardiovascular risk factors are addressed simultaneously. Under the current paradigm of aggressive risk factor modification in patients with diabetes, there is evidence that measures of 10-year coronary heart disease (CHD) risk among U.S. adults with diabetes have improved significantly over the past decade (2) and that ASCVD morbidity and mortality have decreased (3,4).
Heart failure is another major cause of morbidity and mortality from cardiovascular disease. Recent studies have found that rates of incident heart failure hospitalization (adjusted for age and sex) were twofold higher in patients with diabetes compared with those without (5,6). People with diabetes may have heart failure with preserved ejection fraction (HFpEF) or with reduced ejection fraction (HFrEF). Hypertension is often a precursor of heart failure of either type, and ASCVD can coexist with either type (7), whereas prior myocardial infarction (MI) is often a major factor in HFrEF. Rates of heart failure hospitalization have been improved in recent trials including patients with type 2 diabetes, most of whom also had ASCVD, with sodium–glucose cotransporter 2 (SGLT2) inhibitors (8–10).
For prevention and management of both ASCVD and heart failure, cardiovascular risk factors should be systematically assessed at least annually in all patients with diabetes. These risk factors include obesity/overweight, hypertension, dyslipidemia, smoking, a family history of premature coronary disease, chronic kidney disease, and the presence of albuminuria. Modifiable abnormal risk factors should be treated as described in these guidelines. Notably, the majority of evidence supporting interventions to reduce cardiovascular risk in diabetes comes from trials of patients with type 2 diabetes. Few trials have been specifically designed to assess the impact of cardiovascular risk reduction strategies in patients with type 1 diabetes.
The American College of Cardiology/American Heart Association ASCVD risk calculator (Risk Estimator Plus) is generally a useful tool to estimate 10-year risk of a first ASCVD event (available online at tools.acc.org/ASCVD-Risk-Estimator-Plus). The calculator includes diabetes as a risk factor, since diabetes itself confers increased risk for ASCVD, although it should be acknowledged that these risk calculators do not account for the duration of diabetes or the presence of diabetes complications, such as albuminuria. Although some variability in calibration exists in various subgroups, including by sex, race, and diabetes, the overall risk prediction does not differ in those with or without diabetes (11–14), validating the use of risk calculators in people with diabetes. The 10-year risk of a first ASCVD event should be assessed to better stratify ASCVD risk and help guide therapy, as described below.
Recently, risk scores and other cardiovascular biomarkers have been developed for risk stratification of secondary prevention patients (i.e., those who are already high risk because they have ASCVD) but are not yet in widespread use (15,16). With newer, more expensive lipid-lowering therapies now available, use of these risk assessments may help target these new therapies to “higher risk” ASCVD patients in the future.
Hypertension, defined as a sustained blood pressure ≥140/90 mmHg, is common among patients with either type 1 or type 2 diabetes. Hypertension is a major risk factor for both ASCVD and microvascular complications. Moreover, numerous studies have shown that antihypertensive therapy reduces ASCVD events, heart failure, and microvascular complications. Please refer to the American Diabetes Association (ADA) position statement “Diabetes and Hypertension” for a detailed review of the epidemiology, diagnosis, and treatment of hypertension (17).
Blood pressure should be measured at every routine clinical visit by a trained individual and should follow the guidelines established for the general population: measurement in the seated position, with feet on the floor and arm supported at heart level, after 5 min of rest. Cuff size should be appropriate for the upper-arm circumference. Elevated values should be confirmed on a separate day. Postural changes in blood pressure and pulse may be evidence of autonomic neuropathy and therefore require adjustment of blood pressure targets. Orthostatic blood pressure measurements should be checked on initial visit and as indicated.
Home blood pressure self-monitoring and 24-h ambulatory blood pressure monitoring may provide evidence of white coat hypertension, masked hypertension, or other discrepancies between office and “true” blood pressure (17). In addition to confirming or refuting a diagnosis of hypertension, home blood pressure assessment may be useful to monitor antihypertensive treatment. Studies of individuals without diabetes found that home measurements may better correlate with ASCVD risk than office measurements (18,19). Moreover, home blood pressure monitoring may improve patient medication adherence and thus help reduce cardiovascular risk (20).
Randomized controlled trials of intensive versus standard hypertension treatment strategies
| Clinical trial . | Population . | Intensive . | Standard . | Outcomes . |
|---|---|---|---|---|
| ACCORD BP (28) | 4,733 participants with T2D aged 40–79 years with prior evidence of CVD or multiple cardiovascular risk factors | SBP target: | SBP target:130–140 mmHgAchieved (mean) SBP/DBP:13.5/70.5 mmHg | • No benefit in primary end point: composite of nonfatal MI, nonfatal stroke, and CVD death |
| • Stroke risk reduced 41% with intensive control, not sustained through follow-up beyond the period of active treatment | ||||
| • Adverse events more common in intensive group, particularly elevated serum creatinine and electrolyte abnormalities | ||||
| ADVANCE BP (29) | 11,140 participants with T2D aged 55 years and older with prior evidence of CVD or multiple cardiovascular risk factors | Intervention: a single-pill, fixed-dose combination of perindopril and indapamide Achieved (mean) SBP/DBP: 136/73 mmHg | Control: placeboAchieved (mean) SBP/DBP:141.6/75.2 mmHg | • Intervention reduced risk of primary composite end point of major macrovascular and microvascular events (9%), death from any cause (14%), and death from CVD (18%) • 6-year observational follow-up found reduction in risk of death in intervention group attenuated but still significant (198) |
| HOT (199) | 18,790 participants, including 1,501 with diabetes | DBP target:≤80 mmHg | DBP target: ≤90 mmHg | • In the overall trial, there was no cardiovascular benefit with more intensive targets • In the subpopulation with diabetes, an intensive DBP target was associated with a significantly reduced risk (51%) of CVD events |
| SPRINT (40) | 9,361 participants without diabetes | SBP target: | SBP target: | • Intensive SBP target lowered risk of the primary composite outcome 25% (MI, ACS, stroke, heart failure, and death due to CVD) • Intensive target reduced risk of death 27% • Intensive therapy increased risks of electrolyte abnormalities and AKI |
| Clinical trial . | Population . | Intensive . | Standard . | Outcomes . |
|---|---|---|---|---|
| ACCORD BP (28) | 4,733 participants with T2D aged 40–79 years with prior evidence of CVD or multiple cardiovascular risk factors | SBP target: | SBP target:130–140 mmHgAchieved (mean) SBP/DBP:13.5/70.5 mmHg | • No benefit in primary end point: composite of nonfatal MI, nonfatal stroke, and CVD death |
| • Stroke risk reduced 41% with intensive control, not sustained through follow-up beyond the period of active treatment | ||||
| • Adverse events more common in intensive group, particularly elevated serum creatinine and electrolyte abnormalities | ||||
| ADVANCE BP (29) | 11,140 participants with T2D aged 55 years and older with prior evidence of CVD or multiple cardiovascular risk factors | Intervention: a single-pill, fixed-dose combination of perindopril and indapamide Achieved (mean) SBP/DBP: 136/73 mmHg | Control: placeboAchieved (mean) SBP/DBP:141.6/75.2 mmHg | • Intervention reduced risk of primary composite end point of major macrovascular and microvascular events (9%), death from any cause (14%), and death from CVD (18%) • 6-year observational follow-up found reduction in risk of death in intervention group attenuated but still significant (198) |
| HOT (199) | 18,790 participants, including 1,501 with diabetes | DBP target:≤80 mmHg | DBP target: ≤90 mmHg | • In the overall trial, there was no cardiovascular benefit with more intensive targets • In the subpopulation with diabetes, an intensive DBP target was associated with a significantly reduced risk (51%) of CVD events |
| SPRINT (40) | 9,361 participants without diabetes | SBP target: | SBP target: | • Intensive SBP target lowered risk of the primary composite outcome 25% (MI, ACS, stroke, heart failure, and death due to CVD) • Intensive target reduced risk of death 27% • Intensive therapy increased risks of electrolyte abnormalities and AKI |
ACCORD BP, Action to Control Cardiovascular Risk in Diabetes Blood Pressure trial; ACS, acute coronary syndrome; ADVANCE BP, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation–Blood Pressure trial; AKI, acute kidney injury; CVD, cardiovascular disease; DBP, diastolic blood pressure; HOT, Hypertension Optimal Treatment trial; MI, myocardial infarction; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial; T2D, type 2 diabetes. Data from this table can also be found in the ADA position statement “Diabetes and Hypertension” (17).
Additional studies, such as the Systolic Blood Pressure Intervention Trial (SPRINT) and the Hypertension Optimal Treatment (HOT) trial, also examined effects of intensive versus standard control (Table 10.1), though the relevance of their results to people with diabetes is less clear. The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation–Blood Pressure (ADVANCE BP) trial did not explicitly test blood pressure targets (29); the achieved blood pressure in the intervention group was higher than that achieved in the ACCORD BP intensive arm and would be consistent with a target blood pressure of
A number of post hoc analyses have attempted to explain the apparently divergent results of ACCORD BP and SPRINT. Some investigators have argued that the divergent results are not due to differences between people with and without diabetes but rather are due to differences in study design or to characteristics other than diabetes (31–33). Others have opined that the divergent results are most readily explained by the lack of benefit of intensive blood pressure control on cardiovascular mortality in ACCORD BP, which may be due to differential mechanisms underlying cardiovascular disease in type 2 diabetes, to chance, or both (34). Interestingly, a post hoc analysis has found that intensive blood pressure lowering increased the risk of incident chronic kidney disease in both ACCORD BP and SPRINT, with the absolute risk of incident chronic kidney disease being higher in individuals with type 2 diabetes (35).
To clarify optimal blood pressure targets in patients with diabetes, meta-analyses have stratified clinical trials by mean baseline blood pressure or mean blood pressure attained in the intervention (or intensive treatment) arm. Based on these analyses, antihypertensive treatment appears to be beneficial when mean baseline blood pressure is ≥140/90 mmHg or mean attained intensive blood pressure is ≥130/80 mmHg (17,21,22,24–26). Among trials with lower baseline or attained blood pressure, antihypertensive treatment reduced the risk of stroke, retinopathy, and albuminuria, but effects on other ASCVD outcomes and heart failure were not evident. Taken together, these meta-analyses consistently show that treating patients with baseline blood pressure ≥140 mmHg to targets
Patients and clinicians should engage in a shared decision-making process to determine individual blood pressure targets (17). This approach acknowledges that the benefits and risks of intensive blood pressure targets are uncertain and may vary across patients and is consistent with a patient-focused approach to care that values patient priorities and provider judgment (36). Secondary analyses of ACCORD BP and SPRINT suggest that clinical factors can help determine individuals more likely to benefit and less likely to be harmed by intensive blood pressure control (37,38).
Absolute benefit from blood pressure reduction correlated with absolute baseline cardiovascular risk in SPRINT and in earlier clinical trials conducted at higher baseline blood pressure levels (11,38). Extrapolation of these studies suggests that patients with diabetes may also be more likely to benefit from intensive blood pressure control when they have high absolute cardiovascular risk. Therefore, it may be reasonable to target blood pressure
Potential adverse effects of antihypertensive therapy (e.g., hypotension, syncope, falls, acute kidney injury, and electrolyte abnormalities) should also be taken into account (28,35,40,41). Patients with older age, chronic kidney disease, and frailty have been shown to be at higher risk of adverse effects of intensive blood pressure control (41). In addition, patients with orthostatic hypotension, substantial comorbidity, functional limitations, or polypharmacy may be at high risk of adverse effects, and some patients may prefer higher blood pressure targets to enhance quality of life. Patients with low absolute cardiovascular risk (10-year ASCVD risk <15%) or with a history of adverse effects of intensive blood pressure control or at high risk of such adverse effects should have a higher blood pressure target. In such patients, a blood pressure target of <140/90 mmHg is recommended, if it can be safely attained.
There are few randomized controlled trials of antihypertensive therapy in pregnant women with diabetes. A 2014 Cochrane systematic review of antihypertensive therapy for mild to moderate chronic hypertension that included 49 trials and over 4,700 women did not find any conclusive evidence for or against blood pressure treatment to reduce the risk of preeclampsia for the mother or effects on perinatal outcomes such as preterm birth, small-for-gestational-age infants, or fetal death (42). The more recent Control of Hypertension in Pregnancy Study (CHIPS) (43) enrolled mostly women with chronic hypertension. In CHIPS, targeting a diastolic blood pressure of 85 mmHg during pregnancy was associated with reduced likelihood of developing accelerated maternal hypertension and no demonstrable adverse outcome for infants compared with targeting a higher diastolic blood pressure. The mean systolic blood pressure achieved in the more intensively treated group was 133.1 ± 0.5 mmHg, and the mean diastolic blood pressure achieved in that group was 85.3 ± 0.3 mmHg. A similar approach is supported by the International Society for the Study of Hypertension in Pregnancy, which specifically recommends use of antihypertensive therapy to maintain systolic blood pressure between 110 and 140 mmHg and diastolic blood pressure between 80 and 85 mmHg (44). Current evidence supports controlling blood pressure to 110–135/85 mmHg to reduce the risk of accelerated maternal hypertension but also to minimize impairment of fetal growth. During pregnancy, treatment with ACE inhibitors, angiotensin receptor blockers, and spironolactone are contraindicated as they may cause fetal damage. Antihypertensive drugs known to be effective and safe in pregnancy include methyldopa, labetalol, and long-acting nifedipine, while hydralzine may be considered in the acute management of hypertension in pregnancy or severe preeclampsia (45). Diuretics are not recommended for blood pressure control in pregnancy but may be used during late-stage pregnancy if needed for volume control (45,46). The American College of Obstetricians and Gynecologists also recommends that postpartum patients with gestational hypertension, preeclampsia, and superimposed preeclampsia have their blood pressures observed for 72 h in the hospital and for 7–10 days postpartum. Long-term follow-up is recommended for these women as they have increased lifetime cardiovascular risk (47). See Section 14 “Management of Diabetes in Pregnancy” (https://doi.org/10.2337/dc21-S014) for additional information.
Lifestyle management is an important component of hypertension treatment because it lowers blood pressure, enhances the effectiveness of some antihypertensive medications, promotes other aspects of metabolic and vascular health, and generally leads to few adverse effects. Lifestyle therapy consists of reducing excess body weight through caloric restriction (see Section 8 “Obesity Management for the Treatment of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S008), restricting sodium intake (
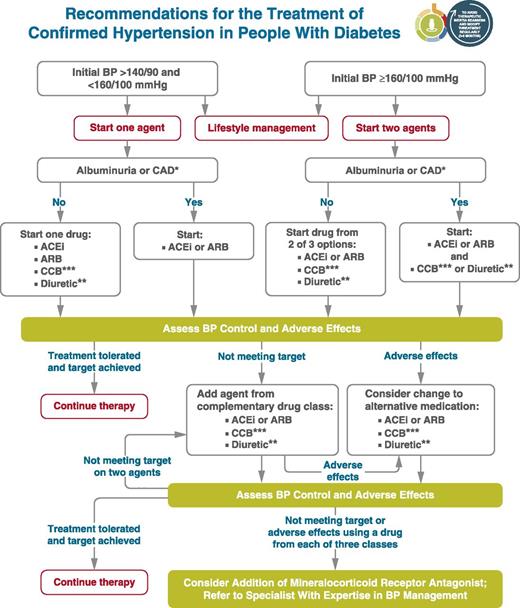
These lifestyle interventions are reasonable for individuals with diabetes and mildly elevated blood pressure (systolic >120 mmHg or diastolic >80 mmHg) and should be initiated along with pharmacologic therapy when hypertension is diagnosed (Fig. 10.1) (49). A lifestyle therapy plan should be developed in collaboration with the patient and discussed as part of diabetes management.
Figure 10.1
Recommendations for the treatment of confirmed hypertension in people with diabetes. *An ACE inhibitor (ACEi) or angiotensin receptor blocker (ARB) is suggested to treat hypertension for patients with coronary artery disease (CAD) or urine albumin-to-creatinine ratio 30–299 mg/g creatinine and strongly recommended for patients with urine albumin-to-creatinine ratio ≥300 mg/g creatinine. **Thiazide-like diuretic; long-acting agents shown to reduce cardiovascular events, such as chlorthalidone and indapamide, are preferred. ***Dihydropyridine calcium channel blocker (CCB). BP, blood pressure. Adapted from de Boer et al. (17).
Figure 10.1
Recommendations for the treatment of confirmed hypertension in people with diabetes. *An ACE inhibitor (ACEi) or angiotensin receptor blocker (ARB) is suggested to treat hypertension for patients with coronary artery disease (CAD) or urine albumin-to-creatinine ratio 30–299 mg/g creatinine and strongly recommended for patients with urine albumin-to-creatinine ratio ≥300 mg/g creatinine. **Thiazide-like diuretic; long-acting agents shown to reduce cardiovascular events, such as chlorthalidone and indapamide, are preferred. ***Dihydropyridine calcium channel blocker (CCB). BP, blood pressure. Adapted from de Boer et al. (17).
Initial treatment for people with diabetes depends on the severity of hypertension (Fig. 10.1). Those with blood pressure between 140/90 mmHg and 159/99 mmHg may begin with a single drug. For patients with blood pressure ≥160/100 mmHg, initial pharmacologic treatment with two antihypertensive medications is recommended in order to more effectively achieve adequate blood pressure control (50–52). Single-pill antihypertensive combinations may improve medication adherence in some patients (53).
Initial treatment for hypertension should include any of the drug classes demonstrated to reduce cardiovascular events in patients with diabetes: ACE inhibitors (54,55), angiotensin receptor blockers (ARBs) (54,55), thiazide-like diuretics (56), or dihydropyridine calcium channel blockers (57). In patients with diabetes and established coronary artery disease, ACE inhibitors or ARBs are recommended first-line therapy for hypertension (58–60). For patients with albuminuria (urine albumin-to-creatinine ratio [UACR] ≥30 mg/g), initial treatment should include an ACE inhibitor or ARB in order to reduce the risk of progressive kidney disease (17) (Fig. 10.1). In the absence of albuminuria, risk of progressive kidney disease is low, and ACE inhibitors and ARBs have not been found to afford superior cardioprotection when compared with thiazide-like diuretics or dihydropyridine calcium channel blockers (61). β-Blockers are indicated in the setting of prior MI, active angina, or HfrEF but have not been shown to reduce mortality as blood pressure–lowering agents in the absence of these conditions (23,62,63).
Multiple-drug therapy is often required to achieve blood pressure targets (Fig. 10.1), particularly in the setting of diabetic kidney disease. However, the use of both ACE inhibitors and ARBs in combination, or the combination of an ACE inhibitor or ARB and a direct renin inhibitor, is not recommended given the lack of added ASCVD benefit and increased rate of adverse events—namely, hyperkalemia, syncope, and acute kidney injury (AKI) (64–66). Titration of and/or addition of further blood pressure medications should be made in a timely fashion to overcome therapeutic inertia in achieving blood pressure targets.
Growing evidence suggests that there is an association between the absence of nocturnal blood pressure dipping and the incidence of ASCVD. A meta-analysis of randomized clinical trials found a small benefit of evening versus morning dosing of antihypertensive medications with regard to blood pressure control but had no data on clinical effects (67). In two subgroup analyses of a single subsequent randomized controlled trial, moving at least one antihypertensive medication to bedtime significantly reduced cardiovascular events, but results were based on a small number of events (68).
Treatment with ACE inhibitors or ARBs can cause AKI and hyperkalemia, while diuretics can cause AKI and either hypokalemia or hyperkalemia (depending on mechanism of action) (69,70). Detection and management of these abnormalities is important because AKI and hyperkalemia each increase the risks of cardiovascular events and death (71). Therefore, serum creatinine and potassium should be monitored during treatment with an ACE inhibitor, ARB, or diuretic, particularly among patients with reduced glomerular filtration who are at increased risk of hyperkalemia and AKI (69,70,72).
Resistant hypertension is defined as blood pressure ≥140/90 mmHg despite a therapeutic strategy that includes appropriate lifestyle management plus a diuretic and two other antihypertensive drugs belonging to different classes at adequate doses. Prior to diagnosing resistant hypertension, a number of other conditions should be excluded, including medication nonadherence, white coat hypertension, and secondary hypertension. In general, barriers to medication adherence (such as cost and side effects) should be identified and addressed (Fig. 10.1). Mineralocorticoid receptor antagonists are effective for management of resistant hypertension in patients with type 2 diabetes when added to existing treatment with an ACE inhibitor or ARB, thiazide-like diuretic, and dihydropyridine calcium channel blocker (73). Mineralocorticoid receptor antagonists also reduce albuminuria and have additional cardiovascular benefits (74–77). However, adding a mineralocorticoid receptor antagonist to a regimen including an ACE inhibitor or ARB may increase the risk for hyperkalemia, emphasizing the importance of regular monitoring for serum creatinine and potassium in these patients, and long-term outcome studies are needed to better evaluate the role of mineralocorticoid receptor antagonists in blood pressure management.
Lifestyle intervention, including weight loss (78), increased physical activity, and medical nutrition therapy, allows some patients to reduce ASCVD risk factors. Nutrition intervention should be tailored according to each patient's age, diabetes type, pharmacologic treatment, lipid levels, and medical conditions.
Recommendations should focus on application of a Mediterranean style diet (79) or Dietary Approaches to Stop Hypertension (DASH) eating pattern, reducing saturated and trans fat intake and increasing plant stanols/sterols, n-3 fatty acids, and viscous fiber (such as in oats, legumes, and citrus) intake (80). Glycemic control may also beneficially modify plasma lipid levels, particularly in patients with very high triglycerides and poor glycemic control. See Section 5 “Facilitating Behavior Change and Well-being to Improve Health Outcomes” (https://doi.org/10.2337/dc21-S005) for additional nutrition information.
In adults with diabetes, it is reasonable to obtain a lipid profile (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides) at the time of diagnosis, at the initial medical evaluation, and at least every 5 years thereafter in patients under the age of 40 years. In younger patients with longer duration of disease (such as those with youth-onset type 1 diabetes), more frequent lipid profiles may be reasonable. A lipid panel should also be obtained immediately before initiating statin therapy. Once a patient is taking a statin, LDL cholesterol levels should be assessed 4–12 weeks after initiation of statin therapy, after any change in dose, and on an individual basis (e.g., to monitor for medication adherence and efficacy). If LDL cholesterol levels are not responding in spite of medication adherence, clinical judgment is recommended to determine the need for and timing of lipid panels. In individual patients, the highly variable LDL cholesterol–lowering response seen with statins is poorly understood (81). Clinicians should attempt to find a dose or alternative statin that is tolerable if side effects occur. There is evidence for benefit from even extremely low, less than daily statin doses (82).
Patients with type 2 diabetes have an increased prevalence of lipid abnormalities, contributing to their high risk of ASCVD. Multiple clinical trials have demonstrated the beneficial effects of statin therapy on ASCVD outcomes in subjects with and without CHD (83,84). Subgroup analyses of patients with diabetes in larger trials (85–89) and trials in patients with diabetes (90,91) showed significant primary and secondary prevention of ASCVD events and CHD death in patients with diabetes. Meta-analyses, including data from over 18,000 patients with diabetes from 14 randomized trials of statin therapy (mean follow-up 4.3 years), demonstrate a 9% proportional reduction in all-cause mortality and 13% reduction in vascular mortality for each 1 mmol/L (39 mg/dL) reduction in LDL cholesterol (92).
Accordingly, statins are the drugs of choice for LDL cholesterol lowering and cardioprotection. Table 10.2 shows the two statin dosing intensities that are recommended for use in clinical practice: high-intensity statin therapy will achieve approximately a ≥50% reduction in LDL cholesterol, and moderate-intensity statin regimens achieve 30–49% reductions in LDL cholesterol. Low-dose statin therapy is generally not recommended in patients with diabetes but is sometimes the only dose of statin that a patient can tolerate. For patients who do not tolerate the intended intensity of statin, the maximally tolerated statin dose should be used.
High-intensity and moderate-intensity statin therapy*
| High-intensity statin therapy (lowers LDL cholesterol by ≥50%) . | Moderate-intensity statin therapy (lowers LDL cholesterol by 30–49%) . |
|---|---|
| Atorvastatin 40–80 mg | Atorvastatin 10–20 mg |
| Rosuvastatin 20–40 mg | Rosuvastatin 5–10 mg |
| Simvastatin 20–40 mg | |
| Pravastatin 40–80 mg | |
| Lovastatin 40 mg | |
| Fluvastatin XL 80 mg | |
| Pitavastatin 1–4 mg |
| High-intensity statin therapy (lowers LDL cholesterol by ≥50%) . | Moderate-intensity statin therapy (lowers LDL cholesterol by 30–49%) . |
|---|---|
| Atorvastatin 40–80 mg | Atorvastatin 10–20 mg |
| Rosuvastatin 20–40 mg | Rosuvastatin 5–10 mg |
| Simvastatin 20–40 mg | |
| Pravastatin 40–80 mg | |
| Lovastatin 40 mg | |
| Fluvastatin XL 80 mg | |
| Pitavastatin 1–4 mg |
*Once-daily dosing. XL, extended release.
As in those without diabetes, absolute reductions in ASCVD outcomes (CHD death and nonfatal MI) are greatest in people with high baseline ASCVD risk (known ASCVD and/or very high LDL cholesterol levels), but the overall benefits of statin therapy in people with diabetes at moderate or even low risk for ASCVD are convincing (93,94). The relative benefit of lipid-lowering therapy has been uniform across most subgroups tested (84,92), including subgroups that varied with respect to age and other risk factors.
For primary prevention, moderate-dose statin therapy is recommended for those 40 years and older (86,93,94), though high-intensity therapy may be considered on an individual basis in the context of additional ASCVD risk factors. The evidence is strong for patients with diabetes aged 40–75 years, an age-group well represented in statin trials showing benefit. Since risk is enhanced in patients with diabetes, as noted above, patients who also have multiple other coronary risk factors have increased risk, equivalent to that of those with ASCVD. As such, recent guidelines recommend that in patients with diabetes who are at higher risk, especially those with multiple ASCVD risk factors or aged 50–70 years, it is reasonable to prescribe high-intensity statin therapy (12,95). Furthermore, for patients with diabetes whose ASCVD risk is ≥20%, i.e., an ASCVD risk equivalent, the same high-intensity statin therapy is recommended as for those with documented ASCVD (12). In those individuals, it may also be reasonable to add ezetimibe to maximally tolerated statin therapy if needed to reduce LDL cholesterol levels by 50% or more (12). The evidence is lower for patients aged >75 years; relatively few older patients with diabetes have been enrolled in primary prevention trials. However, heterogeneity by age has not been seen in the relative benefit of lipid-lowering therapy in trials that included older participants (84,91,92), and because older age confers higher risk, the absolute benefits are actually greater (84,96). Moderate-intensity statin therapy is recommended in patients with diabetes who are 75 years or older. However, the risk-benefit profile should be routinely evaluated in this population, with downward titration of dose performed as needed. See Section 12 “Older Adults” (https://doi.org/10.2337/dc21-S012) for more details on clinical considerations for this population.
Very little clinical trial evidence exists for patients with type 2 diabetes under the age of 40 years or for patients with type 1 diabetes of any age. For pediatric recommendations, see Section 13 “Children and Adolescents” (https://doi.org/10.2337/dc21-S013). In the Heart Protection Study (lower age limit 40 years), the subgroup of ∼600 patients with type 1 diabetes had a proportionately similar, although not statistically significant, reduction in risk as patients with type 2 diabetes (86). Even though the data are not definitive, similar statin treatment approaches should be considered for patients with type 1 or type 2 diabetes, particularly in the presence of other cardiovascular risk factors. Patients below the age of 40 have lower risk of developing a cardiovascular event over a 10-year horizon; however, their lifetime risk of developing cardiovascular disease and suffering an MI, stroke, or cardiovascular death is high. For patients who are younger than 40 years of age and/or have type 1 diabetes with other ASCVD risk factors, it is recommended that the patient and health care provider discuss the relative benefits and risks and consider the use of moderate-intensity statin therapy. Please refer to “Type 1 Diabetes Mellitus and Cardiovascular Disease: A Scientific Statement From the American Heart Association and American Diabetes Association” (97) for additional discussion.
Because risk is high in patients with ASCVD, intensive therapy is indicated and has been shown to be of benefit in multiple large randomized cardiovascular outcomes trials (92,96,98,99). High-intensity statin therapy is recommended for all patients with diabetes and ASCVD. This recommendation is based on the Cholesterol Treatment Trialists' Collaboration involving 26 statin trials, of which 5 compared high-intensity versus moderate-intensity statins. Together, they found reductions in nonfatal cardiovascular events with more intensive therapy, in patients with and without diabetes (84,88,98).
Over the past few years, there have been multiple large randomized trials investigating the benefits of adding nonstatin agents to statin therapy, including those that evaluated further lowering of LDL cholesterol with ezetimibe (96,100) and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (99). Each trial found a significant benefit in the reduction of ASCVD events that was directly related to the degree of further LDL cholesterol lowering. These large trials included a significant number of participants with diabetes. For very high-risk patients with ASCVD who are on high-intensity (and maximally tolerated) statin therapy and have an LDL cholesterol ≥70 mg/dL, the addition of nonstatin LDL-lowering therapy can be considered following a clinician-patient discussion about the net benefit, safety, and cost. Definition of very high-risk patients with ASCVD includes the use of specific criteria (major ASCVD events and high-risk conditions); refer to the 2018 American College of Cardiology/American Heart Association multisociety guideline on the management of blood cholesterol for further details regarding this definition of risk (12).
Please see 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (12) for recommendations for primary and secondary prevention and for statin and combination treatment in adults with diabetes (101).
The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) was a randomized controlled trial in 18,144 patients comparing the addition of ezetimibe to simvastatin therapy versus simvastatin alone. Individuals were ≥50 years of age, had experienced a recent acute coronary syndrome (ACS) and were treated for an average of 6 years. Overall, the addition of ezetimibe led to a 6.4% relative benefit and a 2% absolute reduction in major adverse cardiovascular events (atherosclerotic cardiovascular events), with the degree of benefit being directly proportional to the change in LDL cholesterol, which was 70 mg/dL in the statin group on average and 54 mg/dL in the combination group (96). In those with diabetes (27% of participants), the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) showed a significant reduction of major adverse cardiovascular events with an absolute risk reduction of 5% (40% vs. 45% cumulative incidence at 7 years) and a relative risk reduction of 14% (hazard ratio [HR] 0.86 [95% CI 0.78–0.94]) over moderate-intensity simvastatin (40 mg) alone (100).
Placebo-controlled trials evaluating the addition of the PCSK9 inhibitors evolocumab and alirocumab to maximally tolerated doses of statin therapy in participants who were at high risk for ASCVD demonstrated an average reduction in LDL cholesterol ranging from 36% to 59%. These agents have been approved as adjunctive therapy for patients with ASCVD or familial hypercholesterolemia who are receiving maximally tolerated statin therapy but require additional lowering of LDL cholesterol (102,103).
The effects of PCSK9 inhibition on ASCVD outcomes was investigated in the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial, which enrolled 27,564 patients with prior ASCVD and an additional high-risk feature who were receiving their maximally tolerated statin therapy (two-thirds were on high-intensity statin) but who still had LDL cholesterol ≥70 mg/dL or non-HDL cholesterol ≥100 mg/dL (99). Patients were randomized to receive subcutaneous injections of evolocumab (either 140 mg every 2 weeks or 420 mg every month based on patient preference) versus placebo. Evolocumab reduced LDL cholesterol by 59% from a median of 92 to 30 mg/dL in the treatment arm.
During the median follow-up of 2.2 years, the composite outcome of cardiovascular death, MI, stroke, hospitalization for angina, or revascularization occurred in 11.3% vs. 9.8% of the placebo and evolocumab groups, respectively, representing a 15% relative risk reduction (P < 0.001). The combined end point of cardiovascular death, MI, or stroke was reduced by 20%, from 7.4% to 5.9% (P < 0.001). Importantly, similar benefits were seen in a prespecified subgroup of patients with diabetes, comprising 11,031 patients (40% of the trial) (104).
In the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab), 18,924 patients (28.8% of whom had diabetes) with recent acute coronary syndrome were randomized to the PCSK9 inhibitor alirocumab or placebo every 2 weeks in addition to maximally tolerated statin therapy, with alirocumab dosing titrated between 75 and 150 mg to achieve LDL cholesterol levels between 25 and 50 mg/dL (105).
Over a median follow-up of 2.8 years, a composite primary end point (comprising death from coronary heart disease, nonfatal MI, fatal or nonfatal ischemic stroke, or unstable angina requiring hospital admission) occurred in 903 patients (9.5%) in the alirocumab group and in 1,052 patients (11.1%) in the placebo group (HR 0.85 [95% CI 0.78–0.93]; P < 0.001). Combination therapy with alirocumab plus statin therapy resulted in a greater absolute reduction in the incidence of the primary end point in patients with diabetes (2.3% [95% CI 0.4–4.2]) than in those with prediabetes (1.2% [0.0–2.4]) or normoglycemia (1.2% [–0.3 to 2.7]) (106).
Hypertriglyceridemia should be addressed with dietary and lifestyle changes including weight loss and abstinence from alcohol (107). Severe hypertriglyceridemia (fasting triglycerides ≥500 mg/dL and especially >1,000 mg/dL) may warrant pharmacologic therapy (fibric acid derivatives and/or fish oil) and reduction in dietary fat to reduce the risk of acute pancreatitis. Moderate- or high-intensity statin therapy should also be used as indicated to reduce risk of cardiovascular events (see 1 statin treatment ). In patients with moderate hypertriglyceridemia, lifestyle interventions, treatment of secondary factors, and avoidance of medications that might raise triglycerides are recommended.
The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) enrolled 8,179 adults receiving statin therapy with moderately elevated triglycerides (135–499 mg/dL, median baseline of 216 mg/dL) who had either established cardiovascular disease (secondary prevention cohort) or diabetes plus at least one other cardiovascular risk factor (primary prevention cohort). Patients were randomized to icosapent ethyl 4 g/day (2 g twice daily with food) versus placebo. The trial met its primary end point, demonstrating a 25% relative risk reduction (P < 0.001) for the primary end point composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina. This reduction in risk was seen in patients with or without diabetes at baseline. The composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was reduced by 26% (P < 0.001). Additional ischemic end points were significantly lower in the icosapent ethyl group than in the placebo group, including cardiovascular death, which was reduced by 20% (P = 0.03). The proportions of patients experiencing adverse events and serious adverse events were similar between the active and placebo treatment groups. It should be noted that data are lacking with other n-3 fatty acids, and results of the REDUCE-IT trial should not be extrapolated to other products (108).
Low levels of HDL cholesterol, often associated with elevated triglyceride levels, are the most prevalent pattern of dyslipidemia in individuals with type 2 diabetes. However, the evidence for the use of drugs that target these lipid fractions is substantially less robust than that for statin therapy (109). In a large trial in patients with diabetes, fenofibrate failed to reduce overall cardiovascular outcomes (110).
Combination therapy (statin and fibrate) is associated with an increased risk for abnormal transaminase levels, myositis, and rhabdomyolysis. The risk of rhabdomyolysis is more common with higher doses of statins and renal insufficiency and appears to be higher when statins are combined with gemfibrozil (compared with fenofibrate) (111).
In the ACCORD study, in patients with type 2 diabetes who were at high risk for ASCVD, the combination of fenofibrate and simvastatin did not reduce the rate of fatal cardiovascular events, nonfatal MI, or nonfatal stroke as compared with simvastatin alone. Prespecified subgroup analyses suggested heterogeneity in treatment effects with possible benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L) (112). A prospective trial of a newer fibrate in this specific population of patients is ongoing (113).
The much larger Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) trial also failed to show a benefit of adding niacin to background statin therapy (115). A total of 25,673 patients with prior vascular disease were randomized to receive 2 g of extended-release niacin and 40 mg of laropiprant (an antagonist of the prostaglandin D2 receptor DP1 that has been shown to improve adherence to niacin therapy) versus a matching placebo daily and followed for a median follow-up period of 3.9 years. There was no significant difference in the rate of coronary death, MI, stroke, or coronary revascularization with the addition of niacin–laropiprant versus placebo (13.2% vs. 13.7%; rate ratio 0.96; P = 0.29). Niacin–laropiprant was associated with an increased incidence of new-onset diabetes (absolute excess, 1.3 percentage points; P < 0.001) and disturbances in diabetes control among those with diabetes. In addition, there was an increase in serious adverse events associated with the gastrointestinal system, musculoskeletal system, skin, and, unexpectedly, infection and bleeding.
Therefore, combination therapy with a statin and niacin is not recommended given the lack of efficacy on major ASCVD outcomes and increased side effects.
Several studies have reported a modestly increased risk of incident diabetes with statin use (116,117), which may be limited to those with diabetes risk factors. An analysis of one of the initial studies suggested that although statin use was associated with diabetes risk, the cardiovascular event rate reduction with statins far outweighed the risk of incident diabetes even for patients at highest risk for diabetes (118). The absolute risk increase was small (over 5 years of follow-up, 1.2% of participants on placebo developed diabetes and 1.5% on rosuvastatin developed diabetes) (118). A meta-analysis of 13 randomized statin trials with 91,140 participants showed an odds ratio of 1.09 for a new diagnosis of diabetes, so that (on average) treatment of 255 patients with statins for 4 years resulted in one additional case of diabetes while simultaneously preventing 5.4 vascular events among those 255 patients (117).
Although concerns regarding a potential adverse impact of lipid-lowering agents on cognitive function have been raised, several lines of evidence point against this association, as detailed in a 2018 European Atherosclerosis Society Consensus Panel statement (119). First, there are three large randomized trials of statin versus placebo where specific cognitive tests were performed, and no differences were seen between statin and placebo (120–123). In addition, no change in cognitive function has been reported in studies with the addition of ezetimibe (96) or PCSK9 inhibitors (99,124) to statin therapy, including among patients treated to very low LDL cholesterol levels. In addition, the most recent systematic review of the U.S. Food and Drug Administration's (FDA’s) postmarketing surveillance databases, randomized controlled trials, and cohort, case-control, and cross-sectional studies evaluating cognition in patients receiving statins found that published data do not reveal an adverse effect of statins on cognition (125). Therefore, a concern that statins or other lipid-lowering agents might cause cognitive dysfunction or dementia is not currently supported by evidence and should not deter their use in individuals with diabetes at high risk for ASCVD (125).
Aspirin has been shown to be effective in reducing cardiovascular morbidity and mortality in high-risk patients with previous MI or stroke (secondary prevention) and is strongly recommended. In primary prevention, however, among patients with no previous cardiovascular events, its net benefit is more controversial (126,127).
Previous randomized controlled trials of aspirin specifically in patients with diabetes failed to consistently show a significant reduction in overall ASCVD end points, raising questions about the efficacy of aspirin for primary prevention in people with diabetes, although some sex differences were suggested (128–130).
The Antithrombotic Trialists' Collaboration published an individual patient–level meta-analysis (126) of the six large trials of aspirin for primary prevention in the general population. These trials collectively enrolled over 95,000 participants, including almost 4,000 with diabetes. Overall, they found that aspirin reduced the risk of serious vascular events by 12% (relative risk 0.88 [95% CI 0.82–0.94]). The largest reduction was for nonfatal MI, with little effect on CHD death (relative risk 0.95 [95% CI 0.78–1.15]) or total stroke.
Most recently, the ASCEND (A Study of Cardiovascular Events iN Diabetes) trial randomized 15,480 patients with diabetes but no evident cardiovascular disease to aspirin 100 mg daily or placebo (131). The primary efficacy end point was vascular death, MI, or stroke or transient ischemic attack. The primary safety outcome was major bleeding (i.e., intracranial hemorrhage, sight-threatening bleeding in the eye, gastrointestinal bleeding, or other serious bleeding). During a mean follow-up of 7.4 years, there was a significant 12% reduction in the primary efficacy end point (8.5% vs. 9.6%; P = 0.01). In contrast, major bleeding was significantly increased from 3.2% to 4.1% in the aspirin group (rate ratio 1.29; P = 0.003), with most of the excess being gastrointestinal bleeding and other extracranial bleeding. There were no significant differences by sex, weight, or duration of diabetes or other baseline factors including ASCVD risk score.
Two other large randomized trials of aspirin for primary prevention, in patients without diabetes (ARRIVE [Aspirin to Reduce Risk of Initial Vascular Events]) (132) and in the elderly (ASPREE [Aspirin in Reducing Events in the Elderly]) (133), which included 11% with diabetes, found no benefit of aspirin on the primary efficacy end point and an increased risk of bleeding. In ARRIVE, with 12,546 patients over a period of 60 months follow-up, the primary end point occurred in 4.29% vs. 4.48% of patients in the aspirin versus placebo groups (HR 0.96 [95% CI 0.81–1.13]; P = 0.60). Gastrointestinal bleeding events (characterized as mild) occurred in 0.97% of patients in the aspirin group vs. 0.46% in the placebo group (HR 2.11 [95% CI 1.36–3.28]; P = 0.0007). In ASPREE, including 19,114 individuals, for cardiovascular disease (fatal CHD, MI, stroke, or hospitalization for heart failure) after a median of 4.7 years of follow-up, the rates per 1,000 person-years were 10.7 vs. 11.3 events in aspirin vs. placebo groups (HR 0.95 [95% CI 0.83–1.08]). The rate of major hemorrhage per 1,000 person-years was 8.6 events vs. 6.2 events, respectively (HR 1.38 [95% CI 1.18–1.62]; P < 0.001).
Thus, aspirin appears to have a modest effect on ischemic vascular events, with the absolute decrease in events depending on the underlying ASCVD risk. The main adverse effect is an increased risk of gastrointestinal bleeding. The excess risk may be as high as 5 per 1,000 per year in real-world settings. However, for adults with ASCVD risk >1% per year, the number of ASCVD events prevented will be similar to the number of episodes of bleeding induced, although these complications do not have equal effects on long-term health (134).
Recommendations for using aspirin as primary prevention include both men and women aged ≥50 years with diabetes and at least one additional major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease/albuminuria) who are not at increased risk of bleeding (e.g., older age, anemia, renal disease) (135–138). Noninvasive imaging techniques such as coronary calcium scoring may potentially help further tailor aspirin therapy, particularly in those at low risk (139,140). For patients over the age of 70 years (with or without diabetes), the balance appears to have greater risk than benefit (131,133). Thus, for primary prevention, the use of aspirin needs to be carefully considered and may generally not be recommended. Aspirin may be considered in the context of high cardiovascular risk with low bleeding risk, but generally not in older adults. Aspirin therapy for primary prevention may be considered in the context of shared decision-making, which carefully weighs the cardiovascular benefits with the fairly comparable increase in risk of bleeding. For patients with documented ASCVD, use of aspirin for secondary prevention has far greater benefit than risk; for this indication, aspirin is still recommended (126).
Average daily dosages used in most clinical trials involving patients with diabetes ranged from 50 mg to 650 mg but were mostly in the range of 100–325 mg/day. There is little evidence to support any specific dose, but using the lowest possible dose may help to reduce side effects (142). In the U.S., the most common low-dose tablet is 81 mg. Although platelets from patients with diabetes have altered function, it is unclear what, if any, effect that finding has on the required dose of aspirin for cardioprotective effects in the patient with diabetes. Many alternate pathways for platelet activation exist that are independent of thromboxane A2 and thus are not sensitive to the effects of aspirin (143). “Aspirin resistance” has been described in patients with diabetes when measured by a variety of ex vivo and in vitro methods (platelet aggregometry, measurement of thromboxane B2) (144), but other studies suggest no impairment in aspirin response among patients with diabetes (145). A recent trial suggested that more frequent dosing regimens of aspirin may reduce platelet reactivity in individuals with diabetes (146); however, these observations alone are insufficient to empirically recommend that higher doses of aspirin be used in this group at this time. Another recent meta-analysis raised the hypothesis that low-dose aspirin efficacy is reduced in those weighing more than 70 kg (147); however, the ASCEND trial found benefit of low-dose aspirin in those in this weight range, which would thus not validate this suggested hypothesis (131). It appears that 75–162 mg/day is optimal.
A P2Y12 receptor antagonist in combination with aspirin is reasonable for at least 1 year in patients following an ACS and may have benefits beyond this period. Evidence supports use of either ticagrelor or clopidogrel if no percutaneous coronary intervention was performed and clopidogrel, ticagrelor, or prasugrel if a percutaneous coronary intervention was performed (148). In patients with diabetes and prior MI (1–3 years before), adding ticagrelor to aspirin significantly reduces the risk of recurrent ischemic events including cardiovascular and CHD death (149). Similarly, the addition of ticagrelor to aspirin reduced the risk of ischemic cardiovascular events compared with aspirin alone in patients with diabetes and stable coronary artery disease (150,151). However, a higher incidence of major bleeding, including intracranial hemorrhage, was noted with dual antiplatelet therapy. The net clinical benefit (ischemic benefit vs. bleeding risk) was improved with ticagrelor therapy in the large prespecified subgroup of patients with history of percutaneous coronary intervention, while no net benefit was seen in patients without prior percutaneous coronary intervention (151).
Combination therapy with aspirin plus low dose rivaroxaban may be considered for patients with stable coronary and/or peripheral artery disease to prevent major adverse limb and cardiovascular complications. In the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial of 27,395 patients with established coronary artery disease and/or peripheral artery disease, aspirin plus rivaroxaban 2.5 mg twice daily was superior to aspirin plus placebo in the reduction of cardiovascular ischemic events including major adverse limb events. The absolute benefits of combination therapy appeared larger in patients with diabetes, who comprised 10,341 of the trial participants (152,153). A similar treatment strategy was evaluated in the Vascular Outcomes Study of ASA (acetylsalicylic acid) Along with Rivaroxaban in Endovascular or Surgical Limb Revascularization for Peripheral Artery Disease (VOYAGER PAD) trial (154), in which 6,564 patients with peripheral artery disease who had undergone revascularization were randomly assigned to receive rivaroxaban 2.5 mg twice daily plus aspirin or placebo plus aspirin. Rivaroxaban treatment in this group of patients was also associated with a significantly lower incidence of ischemic cardiovascular events, including major adverse limb events. However, an increased risk of major bleeding was noted with rivaroxaban added to aspirin treatment in both COMPASS and VOYAGER PAD.
The risks and benefits of dual antiplatelet or antiplatelet plus anticoagulant treatment strategies should be thoroughly discussed with eligible patients, and shared decision-making should be used to determine an individually appropriate treatment approach.
Cardiovascular and cardiorenal outcomes trials of available antihyperglycemic medications completed after the issuance of the FDA 2008 guidelines: DPP-4 inhibitors
| . | SAVOR-TIMI 53 (194) (n = 16,492) . | EXAMINE (200) (n = 5,380) . | TECOS (196) (n = 14,671) . | CARMELINA (197,201) (n = 6,979) . | CAROLINA (173,202) (n = 6,042) . |
|---|---|---|---|---|---|
| Intervention | Saxagliptin/placebo | Alogliptin/placebo | Sitagliptin/placebo | Linagliptin/placebo | Linagliptin/glimepiride |
| Main inclusion criteria | Type 2 diabetes and history of or multiple risk factors for CVD | Type 2 diabetes and ACS within 15–90 days before randomization | Type 2 diabetes and preexisting CVD | Type 2 diabetes and high CV and renal risk | Type 2 diabetes and high CV risk |
| A1C inclusion criteria (%) | ≥6.5 | 6.5–11.0 | 6.5–8.0 | 6.5–10.0 | 6.5–8.5 |
| Age (years) †† | 65.1 | 61.0 | 65.4 | 65.8 | 64.0 |
| Race (% White) | 75.2 | 72.7 | 67.9 | 80.2 | 73.0 |
| Sex (% male) | 66.9 | 67.9 | 70.7 | 62.9 | 60.0 |
| Diabetes duration (years)†† | 10.3 | 7.1 | 11.6 | 14.7 | 6.2 |
| Median follow-up (years) | 2.1 | 1.5 | 3.0 | 2.2 | 6.3 |
| Statin use (%) | 78 | 91 | 80 | 71.8 | 64.1 |
| Metformin use (%) | 70 | 66 | 82 | 54.8 | 82.5 |
| Prior CVD/CHF (%) | 78/13 | 100/28 | 74/18 | 57/26.8 | 34.5/4.5 |
| Mean baseline A1C (%) | 8.0 | 8.0 | 7.2 | 7.9 | 7.2 |
| Mean difference in A1C between groups at end of treatment (%) | −0.3^ | −0.3^ | −0.3^ | −0.36^ | 0 |
| Year started/reported | 2010/2013 | 2009/2013 | 2008/2015 | 2013/2018 | 2010/2019 |
| Primary outcome§ | 3-point MACE 1.00 (0.89–1.12) | 3-point MACE 0.96 (95% UL ≤1.16) | 4-point MACE 0.98 (0.89–1.08) | 3-point MACE 1.02 (0.89–1.17) | 3-point MACE 0.98 (0.84–1.14) |
| Key secondary outcome§ | Expanded MACE 1.02 (0.94–1.11) | 4-point MACE 0.95 (95% UL ≤1.14) | 3-point MACE 0.99 (0.89–1.10) | Kidney composite (ESRD, sustained ≥40% decrease in eGFR, or renal death) 1.04 (0.89–1.22) | 4-point MACE 0.99 (0.86–1.14) |
| Cardiovascular death§ | 1.03 (0.87–1.22) | 0.85 (0.66–1.10) | 1.03 (0.89–1.19) | 0.96 (0.81–1.14) | 1.00 (0.81–1.24) |
| MI§ | 0.95 (0.80–1.12) | 1.08 (0.88–1.33) | 0.95 (0.81–1.11) | 1.12 (0.90–1.40) | 1.03 (0.82–1.29) |
| Stroke§ | 1.11 (0.88–1.39) | 0.91 (0.55–1.50) | 0.97 (0.79–1.19) | 0.91 (0.67–1.23) | 0.86 (0.66–1.12) |
| HF hospitalization§ | 1.27 (1.07–1.51) | 1.19 (0.90–1.58) | 1.00 (0.83–1.20) | 0.90 (0.74–1.08) | 1.21 (0.92–1.59) |
| Unstable angina hospitalization§ | 1.19 (0.89–1.60) | 0.90 (0.60–1.37) | 0.90 (0.70–1.16) | 0.87 (0.57–1.31) | 1.07 (0.74–1.54) |
| All-cause mortality§ | 1.11 (0.96–1.27) | 0.88 (0.71–1.09) | 1.01 (0.90–1.14) | 0.98 (0.84–1.13) | 0.91 (0.78–1.06) |
| Worsening nephropathy§|| | 1.08 (0.88–1.32) | — | — | Kidney composite (see above) | __ |
| . | SAVOR-TIMI 53 (194) (n = 16,492) . | EXAMINE (200) (n = 5,380) . | TECOS (196) (n = 14,671) . | CARMELINA (197,201) (n = 6,979) . | CAROLINA (173,202) (n = 6,042) . |
|---|---|---|---|---|---|
| Intervention | Saxagliptin/placebo | Alogliptin/placebo | Sitagliptin/placebo | Linagliptin/placebo | Linagliptin/glimepiride |
| Main inclusion criteria | Type 2 diabetes and history of or multiple risk factors for CVD | Type 2 diabetes and ACS within 15–90 days before randomization | Type 2 diabetes and preexisting CVD | Type 2 diabetes and high CV and renal risk | Type 2 diabetes and high CV risk |
| A1C inclusion criteria (%) | ≥6.5 | 6.5–11.0 | 6.5–8.0 | 6.5–10.0 | 6.5–8.5 |
| Age (years) †† | 65.1 | 61.0 | 65.4 | 65.8 | 64.0 |
| Race (% White) | 75.2 | 72.7 | 67.9 | 80.2 | 73.0 |
| Sex (% male) | 66.9 | 67.9 | 70.7 | 62.9 | 60.0 |
| Diabetes duration (years)†† | 10.3 | 7.1 | 11.6 | 14.7 | 6.2 |
| Median follow-up (years) | 2.1 | 1.5 | 3.0 | 2.2 | 6.3 |
| Statin use (%) | 78 | 91 | 80 | 71.8 | 64.1 |
| Metformin use (%) | 70 | 66 | 82 | 54.8 | 82.5 |
| Prior CVD/CHF (%) | 78/13 | 100/28 | 74/18 | 57/26.8 | 34.5/4.5 |
| Mean baseline A1C (%) | 8.0 | 8.0 | 7.2 | 7.9 | 7.2 |
| Mean difference in A1C between groups at end of treatment (%) | −0.3^ | −0.3^ | −0.3^ | −0.36^ | 0 |
| Year started/reported | 2010/2013 | 2009/2013 | 2008/2015 | 2013/2018 | 2010/2019 |
| Primary outcome§ | 3-point MACE 1.00 (0.89–1.12) | 3-point MACE 0.96 (95% UL ≤1.16) | 4-point MACE 0.98 (0.89–1.08) | 3-point MACE 1.02 (0.89–1.17) | 3-point MACE 0.98 (0.84–1.14) |
| Key secondary outcome§ | Expanded MACE 1.02 (0.94–1.11) | 4-point MACE 0.95 (95% UL ≤1.14) | 3-point MACE 0.99 (0.89–1.10) | Kidney composite (ESRD, sustained ≥40% decrease in eGFR, or renal death) 1.04 (0.89–1.22) | 4-point MACE 0.99 (0.86–1.14) |
| Cardiovascular death§ | 1.03 (0.87–1.22) | 0.85 (0.66–1.10) | 1.03 (0.89–1.19) | 0.96 (0.81–1.14) | 1.00 (0.81–1.24) |
| MI§ | 0.95 (0.80–1.12) | 1.08 (0.88–1.33) | 0.95 (0.81–1.11) | 1.12 (0.90–1.40) | 1.03 (0.82–1.29) |
| Stroke§ | 1.11 (0.88–1.39) | 0.91 (0.55–1.50) | 0.97 (0.79–1.19) | 0.91 (0.67–1.23) | 0.86 (0.66–1.12) |
| HF hospitalization§ | 1.27 (1.07–1.51) | 1.19 (0.90–1.58) | 1.00 (0.83–1.20) | 0.90 (0.74–1.08) | 1.21 (0.92–1.59) |
| Unstable angina hospitalization§ | 1.19 (0.89–1.60) | 0.90 (0.60–1.37) | 0.90 (0.70–1.16) | 0.87 (0.57–1.31) | 1.07 (0.74–1.54) |
| All-cause mortality§ | 1.11 (0.96–1.27) | 0.88 (0.71–1.09) | 1.01 (0.90–1.14) | 0.98 (0.84–1.13) | 0.91 (0.78–1.06) |
| Worsening nephropathy§|| | 1.08 (0.88–1.32) | — | — | Kidney composite (see above) | __ |
—, not assessed/reported; ACS, acute coronary syndrome; CHF, congestive heart failure; CV, cardiovascular; CVD, cardiovascular disease; DPP-4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; GLP-1, glucagon-like peptide 1; HF, heart failure; MACE, major adverse cardiac event; MI, myocardial infarction; UL, upper limit. Data from this table was adapted from Cefalu et al. (203) in the January 2018 issue of Diabetes Care.
Age was reported as means in all trials except EXAMINE, which reported medians; diabetes duration was reported as means in all trials except SAVOR-TIMI 53 and EXAMINE, which reported medians.
Outcomes reported as hazard ratio (95% CI).
Worsening nephropathy is defined as as doubling of creatinine level, initiation of dialysis, renal transplantation, or creatinine >6.0 mg/dL (530 mmol/L) in SAVOR-TIMI 53. Worsening nephropathy was a prespecified exploratory adjudicated outcome in SAVOR-TIMI 53.
Significant difference in A1C between groups (P < 0.05).
Table 10.3BCardiovascular and cardiorenal outcomes trials of available antihyperglycemic medications completed after the issuance of the FDA 2008 guidelines: GLP-1 receptor agonists
| . | ELIXA (183) . | LEADER (178) . | SUSTAIN-6 (179)* . | EXSCEL (184) . | Harmony Outcomes (181) . | REWIND (182) . | PIONEER-6 (180) . |
|---|---|---|---|---|---|---|---|
| (n = 6,068) . | (n = 9,340) . | (n = 3,297) . | (n = 14,752) . | (n = 9,463) . | (n = 9,901) . | (n = 3,183) . | |
| Intervention | Lixisenatide/placebo | Liraglutide/placebo | Semaglutide s.c. injection/placebo | Exenatide QW/placebo | Albiglutide/placebo | Dulaglutide/placebo | Semaglutide oral/placebo |
| Main inclusion criteria | Type 2 diabetes and history of ACS ( | Type 2 diabetes and preexisting CVD, CKD, or HF at ≥50 years of age or CV risk at ≥60 years of age | Type 2 diabetes and preexisting CVD, HF, or CKD at ≥50 years of age or CV risk at ≥60 years of age | Type 2 diabetes with or without preexisting CVD | Type 2 diabetes with preexisting CVD | Type 2 diabetes and prior ASCVD event or risk factors for ASCVD | Type 2 diabetes and high CV risk (age of ≥50 years with established CVD or CKD, or age of ≥60 years with CV risk factors only) |
| A1C inclusion criteria (%) | 5.5–11.0 | ≥7.0 | ≥7.0 | 6.5–10.0 | ≥7.0 | ≤9.5 | None |
| Age (years)†† | 60.3 | 64.3 | 64.6 | 62 | 64.1 | 66.2 | 66 |
| Race (% White) | 75.2 | 77.5 | 83.0 | 75.8 | 84.8 | 75.7 | 72.3 |
| Sex (% male) | 69.3 | 64.3 | 60.7 | 62 | 69.4 | 53.7 | 68.4 |
| Diabetes duration (years)†† | 9.3 | 12.8 | 13.9 | 12 | 13.8 | 10.5 | 14.9 |
| Median follow-up (years) | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 |
| Statin use (%) | 93 | 72 | 73 | 74 | 84.0 | 66 | 85.2 (all lipid-lowering) |
| Metformin use (%) | 66 | 76 | 73 | 77 | 73.6 | 81 | 77.4 |
| Prior CVD/CHF (%) | 100/22 | 81/18 | 60/24 | 73.1/16.2 | 100/20.2 | 32/9 | 84.7/12.2 |
| Mean baseline A1C (%) | 7.7 | 8.7 | 8.7 | 8.0 | 8.7 | 7.4 | 8.2 |
| Mean difference in A1C between groups at end of treatment (%) | −0.3^ | −0.4^ | −0.7 or −1.0^† | −0.53^ | −0.52^ | −0.61^ | −0.7 |
| Year started/reported | 2010/2015 | 2010/2016 | 2013/2016 | 2010/2017 | 2015/2018 | 2011/2019 | 2017/2019 |
| Primary outcome§ | 4-point MACE 1.02 (0.89–1.17) | 3-point MACE 0.87 (0.78–0.97) | 3-point MACE 0.74 (0.58–0.95) | 3-point MACE 0.91 (0.83–1.00) | 3-point MACE 0.78 (0.68–0.90) | 3-point MACE 0.88 (0.79–0.99) | 3-point MACE 0.79 (0.57–1.11) |
| Key secondary outcome§ | Expanded MACE (0.90–1.11) | Expanded MACE 0.88 (0.81–0.96) | Expanded MACE 0.74 (0.62–0.89) | Individual components of MACE (see below) | Expanded MACE (with urgent revascularization for unstable angina) 0.78 (0.69–0.90) CV death or HF hospitalization 0.85 (0.70–1.04) Individual components of MACE (see below) | Composite microvascular outcome (eye or renal outcome) 0.87 (0.79–0.95) | Expanded MACE or HF hospitalization 0.82 (0.61–1.10) |
| Cardiovascular death§ | 0.98 (0.78–1.22) | 0.78 (0.66–0.93) | 0.98 (0.65–1.48) | 0.88 (0.76–1.02) | 0.93 (0.73–1.19) | 0.91 (0.78–1.06) | 0.49 (0.27–0.92) |
| MI§ | 1.03 (0.87–1.22) | 0.86 (0.73–1.00) | 0.74 (0.51–1.08) | 0.97 (0.85–1.10) | 0.75 (0.61–0.90) | 0.96 (0.79–1.15) | 1.18 (0.73–1.90) |
| Stroke§ | 1.12 (0.79–1.58) | 0.86 (0.71–1.06) | 0.61 (0.38–0.99) | 0.85 (0.70–1.03) | 0.86 (0.66–1.14) | 0.76 (0.61–0.95) | 0.74 (0.35–1.57) |
| HF hospitalization§ | 0.96 (0.75–1.23) | 0.87 (0.73–1.05) | 1.11 (0.77–1.61) | 0.94 (0.78–1.13) | — | 0.93 (0.77–1.12) | 0.86 (0.48–1.55) |
| Unstable angina hospitalization§ | 1.11 (0.47–2.62) | 0.98 (0.76–1.26) | 0.82 (0.47–1.44) | 1.05 (0.94–1.18) | — | 1.14 (0.84–1.54) | 1.56 (0.60–4.01) |
| All-cause mortality§ | 0.94 (0.78–1.13) | 0.85 (0.74–0.97) | 1.05 (0.74–1.50) | 0.86 (0.77–0.97) | 0.95 (0.79–1.16) | 0.90 (0.80–1.01) | 0.51 (0.31–0.84) |
| Worsening nephropathy§|| | — | 0.78 (0.67–0.92) | 0.64 (0.46–0.88) | — | — | 0.85 (0.77–0.93) | — |
| . | ELIXA (183) . | LEADER (178) . | SUSTAIN-6 (179)* . | EXSCEL (184) . | Harmony Outcomes (181) . | REWIND (182) . | PIONEER-6 (180) . |
|---|---|---|---|---|---|---|---|
| (n = 6,068) . | (n = 9,340) . | (n = 3,297) . | (n = 14,752) . | (n = 9,463) . | (n = 9,901) . | (n = 3,183) . | |
| Intervention | Lixisenatide/placebo | Liraglutide/placebo | Semaglutide s.c. injection/placebo | Exenatide QW/placebo | Albiglutide/placebo | Dulaglutide/placebo | Semaglutide oral/placebo |
| Main inclusion criteria | Type 2 diabetes and history of ACS ( | Type 2 diabetes and preexisting CVD, CKD, or HF at ≥50 years of age or CV risk at ≥60 years of age | Type 2 diabetes and preexisting CVD, HF, or CKD at ≥50 years of age or CV risk at ≥60 years of age | Type 2 diabetes with or without preexisting CVD | Type 2 diabetes with preexisting CVD | Type 2 diabetes and prior ASCVD event or risk factors for ASCVD | Type 2 diabetes and high CV risk (age of ≥50 years with established CVD or CKD, or age of ≥60 years with CV risk factors only) |
| A1C inclusion criteria (%) | 5.5–11.0 | ≥7.0 | ≥7.0 | 6.5–10.0 | ≥7.0 | ≤9.5 | None |
| Age (years)†† | 60.3 | 64.3 | 64.6 | 62 | 64.1 | 66.2 | 66 |
| Race (% White) | 75.2 | 77.5 | 83.0 | 75.8 | 84.8 | 75.7 | 72.3 |
| Sex (% male) | 69.3 | 64.3 | 60.7 | 62 | 69.4 | 53.7 | 68.4 |
| Diabetes duration (years)†† | 9.3 | 12.8 | 13.9 | 12 | 13.8 | 10.5 | 14.9 |
| Median follow-up (years) | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 |
| Statin use (%) | 93 | 72 | 73 | 74 | 84.0 | 66 | 85.2 (all lipid-lowering) |
| Metformin use (%) | 66 | 76 | 73 | 77 | 73.6 | 81 | 77.4 |
| Prior CVD/CHF (%) | 100/22 | 81/18 | 60/24 | 73.1/16.2 | 100/20.2 | 32/9 | 84.7/12.2 |
| Mean baseline A1C (%) | 7.7 | 8.7 | 8.7 | 8.0 | 8.7 | 7.4 | 8.2 |
| Mean difference in A1C between groups at end of treatment (%) | −0.3^ | −0.4^ | −0.7 or −1.0^† | −0.53^ | −0.52^ | −0.61^ | −0.7 |
| Year started/reported | 2010/2015 | 2010/2016 | 2013/2016 | 2010/2017 | 2015/2018 | 2011/2019 | 2017/2019 |
| Primary outcome§ | 4-point MACE 1.02 (0.89–1.17) | 3-point MACE 0.87 (0.78–0.97) | 3-point MACE 0.74 (0.58–0.95) | 3-point MACE 0.91 (0.83–1.00) | 3-point MACE 0.78 (0.68–0.90) | 3-point MACE 0.88 (0.79–0.99) | 3-point MACE 0.79 (0.57–1.11) |
| Key secondary outcome§ | Expanded MACE (0.90–1.11) | Expanded MACE 0.88 (0.81–0.96) | Expanded MACE 0.74 (0.62–0.89) | Individual components of MACE (see below) | Expanded MACE (with urgent revascularization for unstable angina) 0.78 (0.69–0.90) CV death or HF hospitalization 0.85 (0.70–1.04) Individual components of MACE (see below) | Composite microvascular outcome (eye or renal outcome) 0.87 (0.79–0.95) | Expanded MACE or HF hospitalization 0.82 (0.61–1.10) |
| Cardiovascular death§ | 0.98 (0.78–1.22) | 0.78 (0.66–0.93) | 0.98 (0.65–1.48) | 0.88 (0.76–1.02) | 0.93 (0.73–1.19) | 0.91 (0.78–1.06) | 0.49 (0.27–0.92) |
| MI§ | 1.03 (0.87–1.22) | 0.86 (0.73–1.00) | 0.74 (0.51–1.08) | 0.97 (0.85–1.10) | 0.75 (0.61–0.90) | 0.96 (0.79–1.15) | 1.18 (0.73–1.90) |
| Stroke§ | 1.12 (0.79–1.58) | 0.86 (0.71–1.06) | 0.61 (0.38–0.99) | 0.85 (0.70–1.03) | 0.86 (0.66–1.14) | 0.76 (0.61–0.95) | 0.74 (0.35–1.57) |
| HF hospitalization§ | 0.96 (0.75–1.23) | 0.87 (0.73–1.05) | 1.11 (0.77–1.61) | 0.94 (0.78–1.13) | — | 0.93 (0.77–1.12) | 0.86 (0.48–1.55) |
| Unstable angina hospitalization§ | 1.11 (0.47–2.62) | 0.98 (0.76–1.26) | 0.82 (0.47–1.44) | 1.05 (0.94–1.18) | — | 1.14 (0.84–1.54) | 1.56 (0.60–4.01) |
| All-cause mortality§ | 0.94 (0.78–1.13) | 0.85 (0.74–0.97) | 1.05 (0.74–1.50) | 0.86 (0.77–0.97) | 0.95 (0.79–1.16) | 0.90 (0.80–1.01) | 0.51 (0.31–0.84) |
| Worsening nephropathy§|| | — | 0.78 (0.67–0.92) | 0.64 (0.46–0.88) | — | — | 0.85 (0.77–0.93) | — |
—, not assessed/reported; ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CHF, congestive heart failure; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; GLP-1, glucagon-like peptide 1; HF, heart failure; MACE, major adverse cardiac event; MI, myocardial infarction. Data from this table was adapted from Cefalu et al. (203) in the January 2018 issue of Diabetes Care.
Powered to rule out a hazard ratio of 1.8; superiority hypothesis not prespecified.
Age was reported as means in all trials; diabetes duration was reported as means in all trials except EXSCEL, which reported medians.
A1C change of 0.66% with 0.5 mg and 1.05% with 1 mg dose of semaglutide.
Outcomes reported as hazard ratio (95% CI).
Significant difference in A1C between groups (P < 0.05).
Table 10.3CCardiovascular and cardiorenal outcomes trials of available antihyperglycemic medications completed after the issuance of the FDA 2008 guidelines: SGLT2 inhibitors
| . | EMPA-REG OUTCOME (8) (n = 7,020) . | CANVAS Program (9) (n = 10,142) . | DECLARE-TIMI 58 (176) (n = 17,160) . | CREDENCE (174) (n = 4,401) . | DAPA-HF (177) (n = 4,744; 1,983 with diabetes) . |
|---|---|---|---|---|---|
| Intervention | Empagliflozin/placebo | Canagliflozin/placebo | Dapagliflozin/placebo | Canagliflozin/placebo | Dapagliflozin/placebo |
| Main inclusion criteria | Type 2 diabetes and preexisting CVD | Type 2 diabetes and preexisting CVD at ≥30 years of age or >2 CV risk factors at ≥50 years of age | Type 2 diabetes and established ASCVD or multiple risk factors for ASCVD | Type 2 diabetes and albuminuric kidney disease | NYHA class II, III, or IV heart failure and an ejection fraction ≤40%, with or without diabetes |
| A1C inclusion criteria (%) | 7.0–10.0 | 7.0–10.5 | ≥6.5 | 6.5–12 | __ |
| Age (years)†† | 63.1 | 63.3 | 64.0 | 63 | 66 |
| Race (% White) | 72.4 | 78.3 | 79.6 | 66.6 | 70.3 |
| Sex (% male) | 71.5 | 64.2 | 62.6 | 66.1 | 76.6 |
| Diabetes duration (years)†† | 57% >10 | 13.5 | 11.0 | 15.8 | N/A |
| Median follow-up (years) | 3.1 | 3.6 | 4.2 | 2.6 | 1.5 |
| Statin use (%) | 77 | 75 | 75 (statin or ezetimibe use) | 69 | __ |
| Metformin use (%) | 74 | 77 | 82 | 57.8 | 51.2% (of patients with diabetes) |
| Prior CVD/CHF (%) | 99/10 | 65.6/14.4 | 40/10 | 50.4/14.8 | 100% with CHF |
| Mean baseline A1C (%) | 8.1 | 8.2 | 8.3 | 8.3 | __ |
| Mean difference in A1C between groups at end of treatment (%) | −0.3^‡ | −0.58^ | −0.43^ | −0.31 | N/A |
| Year started/reported | 2010/2015 | 2009/2017 | 2013/2018 | 2017/2019 | 2017/2019 |
| Primary outcome§ | 3-point MACE 0.86 (0.74–0.99) | 3-point MACE 0.86 (0.75–0.97)§ | 3-point MACE 0.93 (0.84–1.03) | ESRD, doubling of creatinine, or death from renal or CV cause 0.70 (0.59–0.82) | Worsening heart failure or death from CV causes 0.74 (0.65–0.85) Results did not differ by diabetes status |
| CV death or HF hospitalization 0.83 (0.73–0.95) | |||||
| Key secondary outcome§ | 4-point MACE 0.89 (0.78–1.01) | All-cause and CV mortality (see below) | Death from any cause 0.93 (0.82–1.04) | CV death or HF hospitalization 0.69 (0.57–0.83) 3-point MACE 0.80 (0.67–0.95) | CV death or HF hospitalization 0.75 (0.65–0.85) |
| Renal composite (≥40% decrease in eGFR rate to | |||||
| Cardiovascular death§ | 0.62 (0.49–0.77) | 0.87 (0.72–1.06) | 0.98 (0.82–1.17) | 0.78 (0.61–1.00) | 0.82 (0.69–0.98) |
| MI§ | 0.87 (0.70–1.09) | 0.89 (0.73–1.09) | 0.89 (0.77–1.01) | __ | __ |
| Stroke§ | 1.18 (0.89–1.56) | 0.87 (0.69–1.09) | 1.01 (0.84–1.21) | __ | __ |
| HF hospitalization§ | 0.65 (0.50–0.85) | 0.67 (0.52–0.87) | 0.73 (0.61–0.88) | 0.61 (0.47–0.80) | 0.70 (0.59–0.83) |
| Unstable angina hospitalization§ | 0.99 (0.74–1.34) | — | — | __ | __ |
| All-cause mortality§ | 0.68 (0.57–0.82) | 0.87 (0.74–1.01) | 0.93 (0.82–1.04) | 0.83 (0.68–1.02) | 0.83 (0.71–0.97) |
| Worsening nephropathy§|| | 0.61 (0.53–0.70) | 0.60 (0.47–0.77) | 0.53 (0.43–0.66) | (See primary outcome) | 0.71 (0.44–1.16) |
| . | EMPA-REG OUTCOME (8) (n = 7,020) . | CANVAS Program (9) (n = 10,142) . | DECLARE-TIMI 58 (176) (n = 17,160) . | CREDENCE (174) (n = 4,401) . | DAPA-HF (177) (n = 4,744; 1,983 with diabetes) . |
|---|---|---|---|---|---|
| Intervention | Empagliflozin/placebo | Canagliflozin/placebo | Dapagliflozin/placebo | Canagliflozin/placebo | Dapagliflozin/placebo |
| Main inclusion criteria | Type 2 diabetes and preexisting CVD | Type 2 diabetes and preexisting CVD at ≥30 years of age or >2 CV risk factors at ≥50 years of age | Type 2 diabetes and established ASCVD or multiple risk factors for ASCVD | Type 2 diabetes and albuminuric kidney disease | NYHA class II, III, or IV heart failure and an ejection fraction ≤40%, with or without diabetes |
| A1C inclusion criteria (%) | 7.0–10.0 | 7.0–10.5 | ≥6.5 | 6.5–12 | __ |
| Age (years)†† | 63.1 | 63.3 | 64.0 | 63 | 66 |
| Race (% White) | 72.4 | 78.3 | 79.6 | 66.6 | 70.3 |
| Sex (% male) | 71.5 | 64.2 | 62.6 | 66.1 | 76.6 |
| Diabetes duration (years)†† | 57% >10 | 13.5 | 11.0 | 15.8 | N/A |
| Median follow-up (years) | 3.1 | 3.6 | 4.2 | 2.6 | 1.5 |
| Statin use (%) | 77 | 75 | 75 (statin or ezetimibe use) | 69 | __ |
| Metformin use (%) | 74 | 77 | 82 | 57.8 | 51.2% (of patients with diabetes) |
| Prior CVD/CHF (%) | 99/10 | 65.6/14.4 | 40/10 | 50.4/14.8 | 100% with CHF |
| Mean baseline A1C (%) | 8.1 | 8.2 | 8.3 | 8.3 | __ |
| Mean difference in A1C between groups at end of treatment (%) | −0.3^‡ | −0.58^ | −0.43^ | −0.31 | N/A |
| Year started/reported | 2010/2015 | 2009/2017 | 2013/2018 | 2017/2019 | 2017/2019 |
| Primary outcome§ | 3-point MACE 0.86 (0.74–0.99) | 3-point MACE 0.86 (0.75–0.97)§ | 3-point MACE 0.93 (0.84–1.03) | ESRD, doubling of creatinine, or death from renal or CV cause 0.70 (0.59–0.82) | Worsening heart failure or death from CV causes 0.74 (0.65–0.85) Results did not differ by diabetes status |
| CV death or HF hospitalization 0.83 (0.73–0.95) | |||||
| Key secondary outcome§ | 4-point MACE 0.89 (0.78–1.01) | All-cause and CV mortality (see below) | Death from any cause 0.93 (0.82–1.04) | CV death or HF hospitalization 0.69 (0.57–0.83) 3-point MACE 0.80 (0.67–0.95) | CV death or HF hospitalization 0.75 (0.65–0.85) |
| Renal composite (≥40% decrease in eGFR rate to | |||||
| Cardiovascular death§ | 0.62 (0.49–0.77) | 0.87 (0.72–1.06) | 0.98 (0.82–1.17) | 0.78 (0.61–1.00) | 0.82 (0.69–0.98) |
| MI§ | 0.87 (0.70–1.09) | 0.89 (0.73–1.09) | 0.89 (0.77–1.01) | __ | __ |
| Stroke§ | 1.18 (0.89–1.56) | 0.87 (0.69–1.09) | 1.01 (0.84–1.21) | __ | __ |
| HF hospitalization§ | 0.65 (0.50–0.85) | 0.67 (0.52–0.87) | 0.73 (0.61–0.88) | 0.61 (0.47–0.80) | 0.70 (0.59–0.83) |
| Unstable angina hospitalization§ | 0.99 (0.74–1.34) | — | — | __ | __ |
| All-cause mortality§ | 0.68 (0.57–0.82) | 0.87 (0.74–1.01) | 0.93 (0.82–1.04) | 0.83 (0.68–1.02) | 0.83 (0.71–0.97) |
| Worsening nephropathy§|| | 0.61 (0.53–0.70) | 0.60 (0.47–0.77) | 0.53 (0.43–0.66) | (See primary outcome) | 0.71 (0.44–1.16) |
—, not assessed/reported; CHF, congestive heart failure; CV, cardiovascular; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HF, heart failure; MACE, major adverse cardiac event; MI, myocardial infarction; SGLT2, sodium–glucose cotransporter 2; NYHA, New York Heart Association. Data from this table was adapted from Cefalu et al. (203) in the January 2018 issue of Diabetes Care.
Age was reported as means in all trials; diabetes duration was reported as means in all trials except EMPA-REG OUTCOME, which reported as percentage of population with diabetes duration >10 years, and DECLARE-TIMI 58, which reported median.
A1C change of 0.30 in EMPA-REG OUTCOME is based on pooled results for both doses (i.e., 0.24% for 10 mg and 0.36% for 25 mg of empagliflozin).
Outcomes reported as hazard ratio (95% CI).
Definitions of worsening nephropathy differed between trials.
Significant difference in A1C between groups (P < 0.05).
Candidates for advanced or invasive cardiac testing include those with 1) typical or atypical cardiac symptoms and 2) an abnormal resting electrocardiogram (ECG). Exercise ECG testing without or with echocardiography may be used as the initial test. In adults with diabetes ≥40 years of age, measurement of coronary artery calcium is also reasonable for cardiovascular risk assessment. Pharmacologic stress echocardiography or nuclear imaging should be considered in individuals with diabetes in whom resting ECG abnormalities preclude exercise stress testing (e.g., left bundle branch block or ST-T abnormalities). In addition, individuals who require stress testing and are unable to exercise should undergo pharmacologic stress echocardiography or nuclear imaging.
The screening of asymptomatic patients with high ASCVD risk is not recommended (155), in part because these high-risk patients should already be receiving intensive medical therapy—an approach that provides similar benefit as invasive revascularization (156,157). There is also some evidence that silent ischemia may reverse over time, adding to the controversy concerning aggressive screening strategies (158). In prospective studies, coronary artery calcium has been established as an independent predictor of future ASCVD events in patients with diabetes and is consistently superior to both the UK Prospective Diabetes Study (UKPDS) risk engine and the Framingham Risk Score in predicting risk in this population (159–161). However, a randomized observational trial demonstrated no clinical benefit to routine screening of asymptomatic patients with type 2 diabetes and normal ECGs (162). Despite abnormal myocardial perfusion imaging in more than one in five patients, cardiac outcomes were essentially equal (and very low) in screened versus unscreened patients. Accordingly, indiscriminate screening is not considered cost-effective. Studies have found that a risk factor–based approach to the initial diagnostic evaluation and subsequent follow-up for coronary artery disease fails to identify which patients with type 2 diabetes will have silent ischemia on screening tests (163,164).
Any benefit of newer noninvasive coronary artery disease screening methods, such as computed tomography calcium scoring and computed tomography angiography, to identify patient subgroups for different treatment strategies remains unproven in asymptomatic patients with diabetes, though research is ongoing. Although asymptomatic patients with diabetes with higher coronary disease burden have more future cardiac events (159,165,166), the role of these tests beyond risk stratification is not clear.
While coronary artery screening methods, such as calcium scoring, may improve cardiovascular risk assessment in people with type 2 diabetes (167), their routine use leads to radiation exposure and may result in unnecessary invasive testing such as coronary angiography and revascularization procedures. The ultimate balance of benefit, cost, and risks of such an approach in asymptomatic patients remains controversial, particularly in the modern setting of aggressive ASCVD risk factor control.
Intensive lifestyle intervention focusing on weight loss through decreased caloric intake and increased physical activity as performed in the Action for Health in Diabetes (Look AHEAD) trial may be considered for improving glucose control, fitness, and some ASCVD risk factors (168). Patients at increased ASCVD risk should receive statin, ACE inhibitor, or ARB therapy if the patient has hypertension, and possibly aspirin, unless there are contraindications to a particular drug class. Clear benefit exists for ACE inhibitor or ARB therapy in patients with diabetic kidney disease or hypertension, and these agents are recommended for hypertension management in patients with known ASCVD (particularly coronary artery disease) (59,60,169). β-Blockers should be used in patients with active angina or HFrEF and for 3 years after MI in patients with preserved left ventricular function (170,171).
In 2008, the FDA issued a guidance for industry to perform cardiovascular outcomes trials for all new medications for the treatment for type 2 diabetes amid concerns of increased cardiovascular risk (172). Previously approved diabetes medications were not subject to the guidance. Recently published cardiovascular outcomes trials have provided additional data on cardiovascular and renal outcomes in patients with type 2 diabetes with cardiovascular disease or at high risk for cardiovascular disease (see Table 10.3A, Table 10.3B, and Table 10.3C). An expanded review of the effects of glucose-lowering therapy in patients with chronic kidney disease is included in Section 11 “Microvascular Complications and Foot Care” (https://doi.org/10.2337/dc21-S011).
Cardiovascular outcomes trials of dipeptidyl peptidase 4 (DPP-4) inhibitors have all, so far, not shown cardiovascular benefits relative to placebo. In addition, the CAROLINA (Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Type 2 Diabetes) study demonstrated noninferiority between a DPP-4 inhibitor, linagliptin, and a sulfonylurea, glimepiride, on cardiovascular outcomes despite lower rates of hypoglycemia in the linagliptin treatment group (173). However, results from other new agents have provided a mix of results.
The BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) was a randomized, double-blind trial that assessed the effect of empagliflozin, an SGLT2 inhibitor, versus placebo on cardiovascular outcomes in 7,020 patients with type 2 diabetes and existing cardiovascular disease. Study participants had a mean age of 63 years, 57% had diabetes for more than 10 years, and 99% had established cardiovascular disease. EMPA-REG OUTCOME showed that over a median follow-up of 3.1 years, treatment reduced the composite outcome of MI, stroke, and cardiovascular death by 14% (absolute rate 10.5% vs. 12.1% in the placebo group, HR in the empagliflozin group 0.86 [95% CI 0.74–0.99]; P = 0.04 for superiority) and cardiovascular death by 38% (absolute rate 3.7% vs. 5.9%, HR 0.62 [95% CI 0.49–0.77]; P < 0.001) (8). The FDA added an indication for empagliflozin to reduce the risk of major adverse cardiovascular death in adults with type 2 diabetes and cardiovascular disease.
Two large outcomes trials of the SGLT2 inhibitor canagliflozin that separately assessed 1) the cardiovascular effects of treatment in patients at high risk for major adverse cardiovascular events, and 2) the impact of canagliflozin therapy on cardiorenal outcomes in patients with diabetes-related chronic kidney disease have been conducted (174). First, the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program integrated data from two trials. The CANVAS trial that started in 2009 was partially unblinded prior to completion because of the need to file interim cardiovascular outcomes data for regulatory approval of the drug (175). Thereafter, the postapproval CANVAS-Renal (CANVAS-R) trial was started in 2014. Combining both of these trials, 10,142 participants with type 2 diabetes were randomized to canagliflozin or placebo and were followed for an average 3.6 years. The mean age of patients was 63 years, and 66% had a history of cardiovascular disease. The combined analysis of the two trials found that canagliflozin significantly reduced the composite outcome of cardiovascular death, MI, or stroke versus placebo (occurring in 26.9 vs. 31.5 participants per 1,000 patient-years; HR 0.86 [95% CI 0.75–0.97]). The specific estimates for canagliflozin versus placebo on the primary composite cardiovascular outcome were HR 0.88 (95% CI 0.75–1.03) for the CANVAS trial and 0.82 (0.66–1.01) for CANVAS-R, with no heterogeneity found between trials. Of note, there was an increased risk of lower-limb amputation with canagliflozin (6.3 vs. 3.4 participants per 1,000 patient-years; HR 1.97 [95% CI 1.41–2.75]) (9). Second, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial randomized 4,401 patients with type 2 diabetes and chronic diabetes-related kidney disease (UACR >300 mg/g and estimated glomerular filtration rate 30 to
The Dapagliflozin Effect on Cardiovascular Events–Thrombosis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial was another randomized, double-blind trial that assessed the effects of dapagliflozin versus placebo on cardiovascular and renal outcomes in 17,160 patients with type 2 diabetes and established ASCVD or multiple risk factors for atherosclerotic cardiovascular disease (176). Study participants had a mean age of 64 years, with ∼40% of study participants having established ASCVD at baseline—a characteristic of this trial that differs from other large cardiovascular trials where a majority of participants had established cardiovascular disease. DECLARE-TIMI 58 met the prespecified criteria for noninferiority to placebo with respect to MACE but did not show a lower rate of MACE when compared with placebo (8.8% in the dapagliflozin group and 9.4% in the placebo group; HR 0.93 [95% CI 0.84–1.03]; P = 0.17). A lower rate of cardiovascular death or hospitalization for heart failure was noted (4.9% vs. 5.8%; HR 0.83 [95% CI 0.73–0.95]; P = 0.005), which reflected a lower rate of hospitalization for heart failure (HR 0.73 [95% CI 0.61–0.88]). No difference was seen in cardiovascular death between groups. Results of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, which assessed the effects of dapagliflozin in patients with established heart failure (177), are described in the 2 glucose-lowering therapies and heart failure section.
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial was a randomized, double-blind trial that assessed the effect of liraglutide, a glucagon-like peptide 1 (GLP-1) receptor agonist, versus placebo on cardiovascular outcomes in 9,340 patients with type 2 diabetes at high risk for cardiovascular disease or with cardiovascular disease. Study participants had a mean age of 64 years and a mean duration of diabetes of nearly 13 years. Over 80% of study participants had established cardiovascular disease. After a median follow-up of 3.8 years, LEADER showed that the primary composite outcome (MI, stroke, or cardiovascular death) occurred in fewer participants in the treatment group (13.0%) when compared with the placebo group (14.9%) (HR 0.87 [95% CI 0.78–0.97]; P < 0.001 for noninferiority; P = 0.01 for superiority). Deaths from cardiovascular causes were significantly reduced in the liraglutide group (4.7%) compared with the placebo group (6.0%) (HR 0.78 [95% CI 0.66–0.93]; P = 0.007) (178). The FDA approved the use of liraglutide to reduce the risk of major adverse cardiovascular events, including heart attack, stroke, and cardiovascular death, in adults with type 2 diabetes and established cardiovascular disease.
Results from a moderate-sized trial of another GLP-1 receptor agonist, semaglutide, were consistent with the LEADER trial (179). Semaglutide is a once-weekly GLP-1 receptor agonist approved by the FDA for the treatment of type 2 diabetes. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) was the initial randomized trial powered to test noninferiority of semaglutide for the purpose of regulatory approval. In this study, 3,297 patients with type 2 diabetes were randomized to receive once-weekly semaglutide (0.5 mg or 1.0 mg) or placebo for 2 years. The primary outcome (the first occurrence of cardiovascular death, nonfatal MI, or nonfatal stroke) occurred in 108 patients (6.6%) in the semaglutide group vs. 146 patients (8.9%) in the placebo group (HR 0.74 [95% CI 0.58–0.95]; P 0.001). More patients discontinued treatment in the semaglutide group because of adverse events, mainly gastrointestinal. The cardiovascular effects of the oral formulation of semaglutide compared with placebo have been assessed in Peptide Innovation for Early Diabetes Treatment (PIONEER) 6, a preapproval trial designed to rule out an unacceptable increase in cardiovascular risk. In this trial of 3,183 patients with type 2 diabetes and high cardiovascular risk followed for a median of 15.9 months, oral semaglutide was noninferior to placebo for the primary composite outcome of cardiovascular death, nonfatal MI, or nonfatal stroke (HR 0.79 [95% CI 0.57–1.11]; P < 0.001 for noninferiority) (180). The cardiovascular effects of this formulation of semaglutide will be further tested in a large, longer-term outcomes trial.
The Harmony Outcomes trial randomized 9,463 patients with type 2 diabetes and cardiovascular disease to once-weekly subcutaneous albiglutide or matching placebo, in addition to their standard care. Over a median duration of 1.6 years, the GLP-1 receptor agonist reduced the risk of cardiovascular death, MI, or stroke to an incidence rate of 4.6 events per 100 person-years in the albiglutide group vs. 5.9 events in the placebo group (HR ratio 0.78, P = 0.0006 for superiority) (181). This agent is not currently available for clinical use.
The Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) trial was a randomized, double-blind, placebo-controlled trial that assessed the effect of the once-weekly GLP-1 receptor agonist dulaglutide versus placebo on MACE in ∼9,990 patients with type 2 diabetes at risk for cardiovascular events or with a history of cardiovascular disease (182). Study participants had a mean age of 66 years and a mean duration of diabetes of ∼10 years. Approximately 32% of participants had history of atherosclerotic cardiovascular events at baseline. After a median follow-up of 5.4 years, the primary composite outcome of nonfatal MI, nonfatal stroke, or death from cardiovascular causes occurred in 12.0% and 13.4% of participants in the dulaglutide and placebo treatment groups, respectively (HR 0.88 [95% CI 0.79–0.99]; P = 0.026). These findings equated to incidence rates of 2.4 and 2.7 events per 100 person-years, respectively. The results were consistent across the subgroups of patients with and without history of CV events. All-cause mortality did not differ between groups (P = 0.067).
The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial studied the once-daily GLP-1 receptor agonist lixisenatide on cardiovascular outcomes in patients with type 2 diabetes who had had a recent acute coronary event (183). A total of 6,068 patients with type 2 diabetes with a recent hospitalization for MI or unstable angina within the previous 180 days were randomized to receive lixisenatide or placebo in addition to standard care and were followed for a median of ∼2.1 years. The primary outcome of cardiovascular death, MI, stroke, or hospitalization for unstable angina occurred in 406 patients (13.4%) in the lixisenatide group vs. 399 (13.2%) in the placebo group (HR 1.2 [95% CI 0.89–1.17]), which demonstrated the noninferiority of lixisenatide to placebo (P < 0.001) but did not show superiority (P = 0.81).
The Exenatide Study of Cardiovascular Event Lowering (EXSCEL) trial also reported results with the once-weekly GLP-1 receptor agonist extended-release exenatide and found that major adverse cardiovascular events were numerically lower with use of extended-release exenatide compared with placebo, although this difference was not statistically significant (184). A total of 14,752 patients with type 2 diabetes (of whom 10,782 [73.1%] had previous cardiovascular disease) were randomized to receive extended-release exenatide 2 mg or placebo and followed for a median of 3.2 years. The primary end point of cardiovascular death, MI, or stroke occurred in 839 patients (11.4%; 3.7 events per 100 person-years) in the exenatide group and in 905 patients (12.2%; 4.0 events per 100 person-years) in the placebo group (HR 0.91 [95% CI 0.83–1.00]; P < 0.001 for noninferiority), but exenatide was not superior to placebo with respect to the primary end point (P = 0.06 for superiority). However, all-cause mortality was lower in the exenatide group (HR 0.86 [95% CI 0.77–0.97]). The incidence of acute pancreatitis, pancreatic cancer, medullary thyroid carcinoma, and serious adverse events did not differ significantly between the two groups.
In summary, there are now numerous large randomized controlled trials reporting statistically significant reductions in cardiovascular events for three of the FDA-approved SGLT2 inhibitors (empagliflozin, canagliflozin, and dapagliflozin) and four FDA-approved GLP-1 receptor agonists (liraglutide, albiglutide [although that agent was removed from the market for business reasons], semaglutide [lower risk of cardiovascular events in a moderate-sized clinical trial but one not powered as a cardiovascular outcomes trial], and dulaglutide). Meta-analyses of the trials reported to date suggest that GLP-1 receptor agonists and SGLT2 inhibitors reduce risk of atherosclerotic major adverse cardiovascular events to a comparable degree in patients with type 2 diabetes and established ASCVD (185). SGLT2 inhibitors also appear to reduce risk of heart failure hospitalization and progression of kidney disease in patients with established ASCVD, multiple risk factors for ASCVD, or diabetic kidney disease (186). In patients with type 2 diabetes and established ASCVD, multiple ASCVD risk factors, or diabetic kidney disease, an SGLT2 inhibitor with demonstrated cardiovascular benefit is recommended to reduce the risk of major adverse cardiovascular events and/or heart failure hospitalization. In patients with type 2 diabetes and established ASCVD or multiple risk factors for ASCVD, a glucagon-like peptide 1 receptor agonist with demonstrated cardiovascular benefit is recommended to reduce the risk of major adverse cardiovascular events. For many patients, use of either an SGLT2 inhibitor or a GLP-1 receptor agonist to reduce cardiovascular risk is appropriate. It is unknown whether use of both classes of drugs will provide an additive cardiovascular outcomes benefit.
As many as 50% of patients with type 2 diabetes may develop heart failure (187). Data on the effects of glucose-lowering agents on heart failure outcomes have demonstrated that thiazolidinediones have a strong and consistent relationship with increased risk of heart failure (188–190). Therefore, thiazolidinedione use should be avoided in patients with symptomatic heart failure. Restrictions to use of metformin in patients with medically treated heart failure were removed by the FDA in 2006 (191). In fact, observational studies of patients with type 2 diabetes and heart failure suggest that metformin users have better outcomes than patients treated with other antihyperglycemic agents (192). Metformin may be used for the management of hyperglycemia in patients with stable heart failure as long as kidney function remains within the recommended range for use (193).
Recent studies examining the relationship between DPP-4 inhibitors and heart failure have had mixed results. The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus – Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) study showed that patients treated with the DPP-4 inhibitor saxagliptin were more likely to be hospitalized for heart failure than those given placebo (3.5% vs. 2.8%, respectively) (194). However, three other cardiovascular outcomes trials—Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) (195), Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) (196), and the Cardiovascular and Renal Microvascular Outcome Study With Linagliptin (CARMELINA) (197)—did not find a significant increase in risk of heart failure hospitalization with DPP-4 inhibitor use compared with placebo. No increased risk of heart failure hospitalization has been identified in the cardiovascular outcomes trials of the GLP-1 receptor agonists lixisenatide, liraglutide, semaglutide, exenatide once-weekly, albiglutide, or dulaglutide compared with placebo (Table 10.3B) (178,179,182–184).
Reduced incidence of heart failure has been observed with the use of SGLT2 inhibitors (174,176). In EMPA-REG OUTCOME, the addition of empagliflozin to standard care led to a significant 35% reduction in hospitalization for heart failure compared with placebo (8). Although the majority of patients in the study did not have heart failure at baseline, this benefit was consistent in patients with and without a history of heart failure (10). Similarly, in CANVAS and DECLARE-TIMI 58, there were 33% and 27% reductions in hospitalization for heart failure, respectively, with SGLT2 inhibitor use versus placebo (9,176). Additional data from the CREDENCE trial with canagliflozin showed a 39% reduction in hospitalization for heart failure, and 31% reduction in the composite of cardiovascular death or hospitalization for heart failure, in a diabetic kidney disease population with albuminuria (UACR of >300 to 5,000 mg/g) (174). These combined findings from four large outcomes trials of three different SGLT2 inhibitors are highly consistent and clearly indicate robust benefits of SGLT2 inhibitors in the prevention of heart failure hospitalizations. The EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58, and CREDENCE trials suggested, but did not prove, that SGLT2 inhibitors would be beneficial in the treatment of patients with established heart failure. More recently, the placebo-controlled DAPA-HF trial evaluated the effects of dapagliflozin on the primary outcome of a composite of worsening heart failure or cardiovascular death in patients with New York Heart Association class II, III, or IV heart failure and an ejection fraction of 40% or less. Of the 4,744 trial participants, 45% had a history of type 2 diabetes. Over a median of 18.2 months, the group assigned to dapagliflozin treatment had a lower risk of the primary outcome (HR 0.74 [95% CI 0.65–0.85]), lower risk of first worsening heart failure event (HR 0.70 [95% CI 0.59–0.83]), and lower risk of cardiovascular death (HR 0.82 [95% CI 0.69–0.98]) compared with placebo. The effect of dapagliflozin on the primary outcome was consistent regardless of the presence or absence of type 2 diabetes (177). Therefore, in patients with type 2 diabetes and established HFrEF, an SGLT2 inhibitor with proven benefit in this patient population is recommended to reduce the risk of worsening heart failure and cardiovascular death. The benefits seen in this patient population may represent a class effect. Ongoing trials are assessing the effects of several SGLT2 inhibitors in heart failure patients with both reduced and preserved ejection fraction.
This section has received endorsement from the American College of Cardiology.
Suggested citation: American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl.1):S125–S150